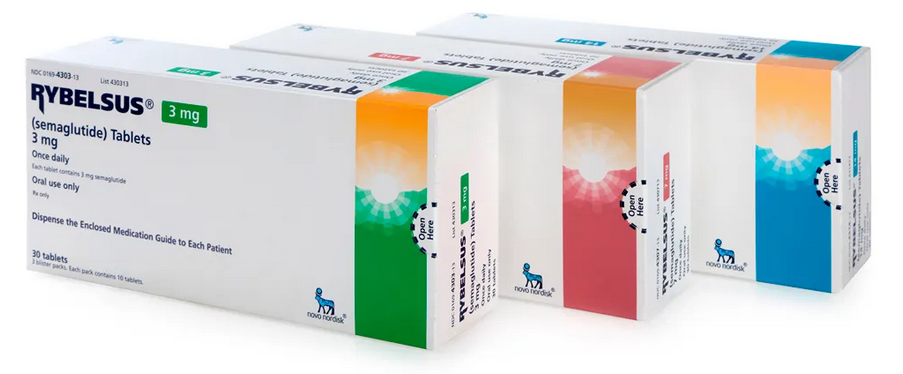
Buy Rybelsus® Semaglutide Tablets Online
- Brand: Rybelsus®
- Composition: Semaglutide
- Power: 3mg, 7mg, 14mg
- Drug form: Tablets
- Disease/Treatment: used in adults with type 2 diabetes mellitus to decrease blood sugar levels
- Manufacturer: Novo Nordisc India Pvt. Ltd
- Country of Origin: India
Order Rybelsus® Semaglutide Tablets Tablets
Rybelsus® Semaglutide Tablets is a hypoglycemic drug that is an analogue of the natural hormone glucagon—like peptide-1, the drug provides an opportunity to carry out insulin-free therapy for patients with type II diabetes, effectively and safely reduces blood sugar levels in patients with type II diabetes.
Rybelsus® Semaglutide Tablets slows down the digestive process, prevents the liver from synthesizing large amounts of sugar, helps the pancreas produce more insulin when needed.
Rybelsus® Semaglutide Tablets can be used for monotherapy of patients, as well as used in combination with other methods of treating diabetes mellitus.
The medicine Rybelsus® Semaglutide Tablets can be purchased at an online pharmacy at a bargain price with delivery.
Composition
1 tablet of the drug contains:
- active substance: semaglutide (3 mg; 7 mg or 14 mg)
- excipients: sodium sucrose, povidone K90, microcrystalline cellulose, magnesium stearate.
Semaglutide is an analog of human glucagon-like peptide-1 (GLP-1) produced by recombinant DNA biotechnology using the Saccharomyces cerevisiae strain.
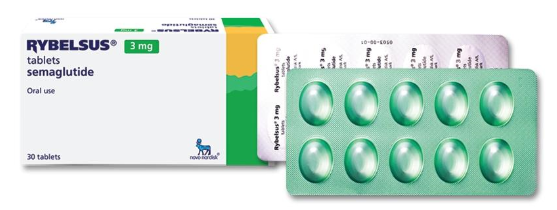
Rybelsus® 3 mg:
Oval biconvex tablets from white to light yellow with the engraving “3” on one side and “novo” on the other.
10 tablets in a blister of molded green aluminum foil laminated with polypropylene and a cover aluminum foil laminated with polypropylene.
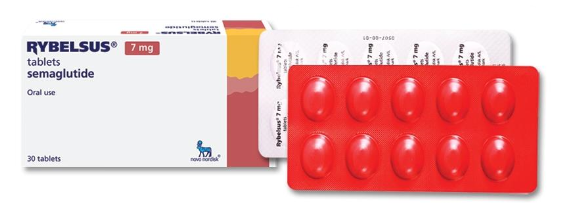
Rybelsus® 7 mg:
Oval biconvex tablets from white to light yellow with the engraving “7” on one side and “novo” on the other.
10 tablets in a blister of molded green aluminum foil laminated with polypropylene and a cover aluminum foil laminated with polypropylene.
Rybelsus® 14 mg:
Oval biconvex tablets from white to light yellow with the engraving “14” on one side and “novo” on the other.
10 tablets in a blister of molded green aluminum foil laminated with polypropylene and a cover aluminum foil laminated with polypropylene.
Pharmacotherapeutic group
Hypoglycemic agent is an analog of glucagon–like peptide-1 (GLP-1).
Description of Rybelsus® Semaglutide Tablets
The proven effectiveness of Rybelsus® Semaglutide Tablets is confirmed by the results of many studies, the drug is part of the standard protocols for the treatment of patients with type II diabetes in the USA, Canada, UK, Australia and other countries of the world. The drug Rybelsus® contains a physiological hormone that regulates the concentration of glucose in the blood, normalizes the work of the CCC, appetite, promotes weight loss. Rybelsus® Semaglutide should be used under the strict supervision of a doctor who has sufficient experience in the treatment of patients with type 2 diabetes mellitus. The drug Rybelsus® Semaglutide causes a persistent response of the body, improves the condition of patients.
The drug Rybelsus® Semaglutide Tablets can be purchased in the USA, Canada, UK, Australia at a competitive price in an online pharmacy by ordering the product and arranging delivery.
The use is contraindicated for patients with type I diabetes, for the treatment of diabetic ketoacidosis. The drug Rybelsus® Semaglutide Tablets does not replace insulin. Before use, it is necessary to read the instructions for the hypoglycemic drug Rybelsus®.
Pharmacodynamics
The pharmacodynamic results of semaglutide for oral administration were evaluated after 12 weeks of use.
Fasting glycemia and postprandial glycemia
Semaglutide reduces fasting glucose concentration and postprandial glucose concentration. In patients with type 2 diabetes mellitus (DM2), semaglutide therapy led to a decrease in fasting glucose concentration by 22% and postprandial glucose concentration by 29% compared with placebo.
Glucagon secretion
Semaglutide reduces the postprandial concentration of glucagon. In patients with DM2, semaglutide leads to a decrease in the concentration of glucagon compared to placebo, namely, to a decrease in the postprandial glucagon response by 29%.
Emptying the stomach
Semaglutide causes a slight delay in early postprandial gastric emptying (paracetamol exposure (AUC0-1h) decreases by 31% in the first hour after eating), thereby reducing the rate of postprandial glucose intake into the blood.
The concentration of lipids on an empty stomach and after meals
Compared with placebo, semaglutide reduced the concentrations of triglycerides and very low density lipoprotein cholesterol (VLDL) on an empty stomach by 19% and 20%, respectively. The postprandial increase in the concentration of triglycerides and VLDL cholesterol in response to a high-fat meal decreased by 24% and 21%, respectively. The concentration of ApoV48 decreased both on an empty stomach and after eating by 25% and 30%, respectively.
Clinical efficacy and safety
The efficacy and safety of Rybelsus® were evaluated in eight global randomized controlled clinical trials (CI) of phase IIIa. Of these, seven CI assessed the effectiveness of glycemic control as the main goal, while one CI assessed the cardiovascular outcome as the main goal.
The CI included 8842 randomized patients with DM2 (5169 people received semaglutide), including 1165 patients with moderate renal impairment. The average age of patients was 61 years (from 18 to 92 years), and 40% of patients were aged 65 years and older, and 8% were 75 years and older. The efficacy of semaglutide was compared with placebo or active control (sitagliptin, empagliflozin and liraglutide).
Age, gender, race, ethnicity, body weight, body mass index (BMI), duration of diabetes mellitus (DM), diseases of the upper gastrointestinal tract and renal insufficiency did not affect the effectiveness of semaglutide.
Monotherapy
In a 26-week double-blind study, 703 patients with DM2 insufficiently controlled by diet and exercise were randomized to the Rybelsus® therapy groups at doses of 3 mg, 7 mg, 14 mg or placebo once a day.
According to the results of the study (data analysis of 703 patients), monotherapy with Rybelsus® at doses of 3 mg, 7 mg and 14 mg once a day for 26 weeks led to a statistically significant decrease in HbA1c by 0.9%, 1.2% and 1.4% compared with 0.3% in the placebo group (p <0.001 for all dosages).
Fasting plasma glucose (GPN) against the background of monotherapy with Rybelsus® at doses of 3 mg, 7 mg and 14 mg once a day for 26 weeks decreased by 0.9 mmol/l, 1.5 mmol/l and 1.8 mmol/l, respectively, compared with 0.2 mmol/l in the placebo group (p=0.003, p<0.001, p<0.001, respectively, doses).
On therapy with Rybelsus® 14 mg, a statistically significant decrease in body weight by 3.7 kg was obtained against a decrease by 1.4 kg in the placebo group (p<0.001). The proportion of patients who reached the target HbA1c <7% was 55%, 69%, 77% and 31% for Rybelsus® 3 mg, 7 mg, 14 mg and placebo, respectively.
Rybelsus® Semaglutide compared to empagliflozin, both in combination with metformin
In a 52-week open CI, 822 patients with DM2 were randomized to therapy groups with Rybelsus® 14 mg once a day or empagliflozin 25 mg once a day, both in combination with metformin.
At week 26 (primary endpoint – data analysis of 822 patients), therapy with Rybelsus® 14 mg once a day compared with empagliflozin 25 mg once a day resulted in a statistically significant decrease in HbA1c by 1.3% versus a decrease of 0.9%, respectively (p <0.0001).
The proportion of patients who reached the target value of HbA1c <7% at week 26 was 67% and 40% for Rybelsus® 14 mg and empagliflozin 25 mg, respectively. The proportion of patients who reached the target value of HbA1c <7% by the end of the study at 52 weeks remained approximately in the same ratio of 66% and 43% for Rybelsus® 14 mg and empagliflozin 25 mg, respectively.
Rybelsus® Semaglutide compared to sitagliptin, both in combination with metformin, or metformin together with sulfonylurea derivatives
In a 78-week double-blind double-masking study, 1,864 patients with DM2 were randomized to therapy groups with Rybelsus® at a dose of 3 mg, 7 mg, 14 mg or sitagliptin at a dose of 100 mg once a day (both in combination with metformin or metformin together with sulfonylurea derivatives).
At week 26 (primary endpoint – data analysis of 1397 patients), therapy with Rybelsus® 7 mg and 14 mg once a day resulted in a statistically significant decrease in HbA1c by 1.0% and 1.3% compared with a decrease of 0.8% on sitagliptin therapy of 100 mg once a day (p<0.001 for both doses, respectively).
The GPN index during therapy with Rybelsus® at doses of 7 mg and 14 mg once a day for 26 weeks decreased by 1.2 mmol/l and 1.7 mmol/l, respectively, compared with 0.9 mmol/l in the sitagliptin 100 mg therapy group once a day (p<0.05, p<0.001, respectively, doses).
During therapy with Rybelsus® 7 mg and 14 mg for 26 weeks, a statistically significant decrease in body weight by 2.2 kg and 3.1 kg was obtained against a decrease of 0.6 kg in the sitagliptin therapy group of 100 mg once a day (p<0.001 for both doses).
The proportion of patients who reached the target value of HbA1c <7% at week 26 of the study was 44%, 56% and 32% for Rybelsus® 7 mg, 14 mg and sitagliptin 100 mg, respectively. The proportion of patients who reached the target value of HbA1c <7% by the end of the study at week 78 remained approximately the same ratio of 39%, 45% and 29% for Rybelsus® 7 mg, 14 mg and sitagliptin 100 mg, respectively.
Rybelsus® Semaglutide compared to liraglutide and placebo, all in combination with metformin or metformin with a type 2 sodium-dependent glucose transporter inhibitor (SGLT2)
In a 52-week double-blind, double-masked study, 711 patients with DM2 were randomized to therapy groups with Rybelsus® 14 mg, liraglutide 1.8 mg as subcutaneous injection or placebo once a day (all in combination with metformin or metformin with an SGLT2 inhibitor).
At week 26 (primary endpoint – data analysis of 711 patients), therapy with Rybelsus® 14 mg once a day resulted in a statistically significant decrease in the HbA1c index by 1.2% compared with a decrease of 1.1% in the liraglutide therapy group of 1.8 mg in the form of subcutaneous injection once a day (p<0.0001 in relation to not less effective) and compared with a decrease of 0.2% in the placebo group (p<0.0001).
The GPN index during therapy with Rybelsus® 14 mg once a day for 26 weeks decreased by 2.0 mmol/l compared with 0.4 mmol/l in the placebo group (p<0.0001).
During therapy with Rybelsus® 14 mg for 26 weeks, a statistically significant decrease in body weight by 4.4 kg was obtained against a decrease of 3.1 kg in the liraglutide therapy group of 1.8 mg in the form of subcutaneous injection once a day (p=0.0003) and compared with a decrease of 0.5 kg in the placebo group (p<0.0001).
The proportion of patients who achieved HbA1c <7% at week 26 of the study was 68%, 62% and 14% for Rybelsus® 14 mg, liraglutide 1.8 mg and placebo, respectively. The proportion of patients who reached the target value of HbA1c <7% by the end of the study at 52 weeks was 61%, 55% and 15% for Rybelsus® 14 mg, liraglutide 1.8 mg and placebo, respectively.
Rybelsus® Semaglutide compared to placebo, both in combination with monotherapy with basal insulin or metformin, or sulfonylurea derivatives, or with a combination of metformin and basal insulin or with a combination of metformin and sulfonylurea derivatives in patients with moderate renal impairment
In a 26-week double-blind study, 324 patients with DM2 and moderate renal impairment (eGFR 30-59 ml/min/1.73 m2) were randomly assigned to the treatment groups with Rybelsus® 14 mg or placebo once a day. The study drug was added to a stable hypoglycemic therapy regimen.
At week 26 (primary endpoint – data analysis of 324 patients), therapy with Rybelsus® 14 mg once a day compared with placebo resulted in a statistically significant decrease in HbA1c by 1.0% compared with 0.2% in the placebo group (p<0.0001). The GPN index during therapy with Rybelsus® 14 mg once a day for 26 weeks decreased by 1.5 mmol/l compared with 0.4 mmol/l in the placebo group (p<0.05).
On therapy with Rybelsus® 14 mg, a statistically significant decrease in body weight by 3.4 kg was obtained compared with a decrease of 0.9 kg in the placebo group (p<0.0001).
The proportion of patients who achieved HbA1c <7% was 58% and 23% for Rybelsus® 14 mg and placebo, respectively.
Rybelsus® Semaglutide compared to sitagliptin, both in combination with metformin, SGLT2 inhibitors, sulfonylurea derivatives or thiazolidinediones.
In a 52-week open-label study, 504 patients with DM2 were randomized to therapy groups with Rybelsus® (flexible dose adjustment of 3 mg, 7 mg and 14 mg once a day) or sitagliptin at a dose of 100 mg once a day, both in combination with 1-2 oral hypoglycemic drugs (metformin, SGLT2 inhibitors, sulfonylurea derivatives or thiazolidinediones). The dose of Rybelsus® was adjusted every 8 weeks depending on the patient’s glycemic response and its tolerability. The dose of sitagliptin 100 mg was fixed.
At week 52 (the primary endpoint is the analysis of data from 504 patients), the number of patients receiving semaglutide therapy at doses of 3 mg, 7 mg and 14 mg was approximately 10%, 30% and 60%, respectively. The proportion of patients who reached the target value of HbA1c < 7% was 58% and 25% for a flexible dose of Rybelsus® once a day and sitagliptin at a fixed dose of 100 mg, respectively (p<0.001). The chance of achieving the target value of HbA1c < 7% for a flexible dose of Rybelsus® once a day was 4.4 times greater than the chance of therapy with sitagliptin at a fixed dose of 100 mg (p<0.0001).
Therapy with a flexible dose of Rybelsus® once a day resulted in a statistically significant decrease in body weight by 2.6 kg compared with a decrease of 0.7 kg in the sitagliptin therapy group at a fixed dose of 100 mg (p<0.001).
Rybelsus® Semaglutide compared to placebo in combination with insulin
In a 52-week double-blind study (the main final indicator at week 26), 731 patients with DM2 insufficiently controlled on insulin therapy (basal, basal/bolus or biphasic) with or without metformin were randomized to the Rybelsus® therapy groups at doses of 3 mg, 7 mg, 14 mg or placebo once a day.
At week 26 (primary endpoint – data analysis of 731 patients), Rybelsus® therapy with 3 mg, 7 mg and 14 mg resulted in a statistically significant decrease in HbA1c by 0.6%, 0.9% and 1.3% compared with 0.1% in the placebo group (p<0.0001 for all doses).
The GPN index in the Rybelsus® therapy group at doses of 7 mg and 14 mg once a day for 26 weeks decreased by 1.1 mmol/l and 1.3 mmol/l, respectively, compared with an increase of 0.3 mmol/l in the placebo group (p<0.0001 for both doses).
During therapy with Rybelsus® 3 mg, 7 mg, 14 mg for 26 weeks, a statistically significant decrease in body weight by 1.4 kg, 2.4 kg and 3.7 kg was obtained against a decrease of 0.4 kg in the placebo group (p<0.05, p<0.001, p<0.001, respectively, dose).
The proportion of patients who reached the target HbA1c <7% at week 26 of the study was 28%, 43%, 58% and 7% for Rybelsus® 3 mg, 7 mg, 14 mg and placebo, respectively. The proportion of patients who reached the target value of HbA1c <7% by the end of the study at 52 weeks remained approximately in the same ratio of 29%, 40%, 54% and 9% for Rybelsus® 3 mg, 7 mg, 14 mg and placebo, respectively.
Assessment of the effect on the cardiovascular system
3183 patients with DM2 and high cardiovascular (CC) risk were randomized to double-blind CI in the treatment groups with Rybelsus® 14 mg once a day or placebo in addition to standard treatment. Taking into account the previously conducted study on the effect on the cardiovascular system (CCC) with respect to the injectable form of semaglutide, the study protocol was aimed at confirming the safety of therapy with Rybelsus® 14 mg, and therefore the study was limited in time: the median follow-up period was 16 months.
The primary endpoint was the time from randomization to the first major cardiovascular event, such as death due to SS pathology, non-fatal myocardial infarction, non-fatal stroke.
Patients eligible for inclusion in the study were aged 50 years or older and with established cardiovascular disease (CVD) and/or chronic kidney disease, or aged 60 years or older with only risk factors for CVD. A total of 1,797 patients (56.5%) had CVD without chronic kidney disease, 354 (11.1%) had only chronic kidney disease and 544 (17.1%) had both CVD and kidney disease. 488 patients (15.3%) had only CVD risk factors. The average age at the time of inclusion in the study was 66 years, 68% of patients were men. The average DM experience was 14.9 years, the average BMI was 32.3 kg/m2. Stroke or myocardial infarction was in the anamnesis in 11.7% and 36.1% of patients, respectively.
The total number of the first BSSS was 137: 61 (3.8%) when using semaglutide and 76 (4.8%) when using placebo, thus, patients in the Rybelsus® therapy group demonstrated a numerical decrease of 21% in the frequency of BSSS compared to patients in the placebo group, the risk ratio was 0.79 (0.57, 1.11), what achieved the initial goal of the study was to demonstrate the absence of an increase in cardiovascular risk on Rybelsus® therapy (p<0.001).
The results of the analysis with respect to the components of the primary endpoint were as follows: death due to SS pathology – 0.9% of cases with semaglutide and 1.9% of cases with placebo (51% decrease in the frequency of events); myocardial infarction without fatal outcome – 2.3% of cases with semaglutide and 1.9% of cases with placebo (no differences in favor of semaglutide); stroke without fatal outcome – 0.8% of cases with semaglutide and 1.0% of cases with placebo (numerical decrease in the frequency of events by 26%). Death for any reason occurred in 1.4% of patients in the semaglutide group and in 2.8% of patients in the placebo group (a 49% decrease in the frequency of events).
Body weight
On semaglutide therapy, by the end of the study, 27-45% of patients had achieved a decrease in body weight by ≥5%, and 6-16% had achieved a decrease in body weight by ≥10% when using semaglutide, compared with 12-39% and 2-8%, respectively, when using active comparison drugs.
Blood pressure
Therapy with Rybelsus® resulted in a decrease in systolic blood pressure by 2-7 mmHg.
Children and teenagers
Safety and efficacy of Rybelsus® in children and adolescents (under 18 years of age) not installed.
What are the Side effects of Rybelsus®?
When using the drug Rybelsus®, the following side effects are possible:
- anaphylactic reactions;
- decreased appetite;
- dizziness, headache;
- gastrointestinal disorders;
- complications of diabetic retinopathy;
- increased activity of lipase, amylase, weight loss.
The use of the drug Rybelsus® according to the instructions is sometimes associated with the development of side effects that pass after the start of appropriate treatment or discontinuation of the Rybelsus® Tablets.
What are the contraindications of Rybelsus?
- Hypersensitivity to semaglutide or to any of the excipients of the drug;
- type 1 diabetes mellitus (DM1);
- diabetic ketoacidosis;
- medullary thyroid cancer in the anamnesis, including family;
- multiple endocrine neoplasia (MEN) type 2;
The use of Rybelsus® Tablets is contraindicated in the following groups of patients and in the following conditions / diseases due to the lack of data on efficacy and safety and / or due to limited experience:
- pregnancy and breastfeeding;
- age under 18;
- severe hepatic insufficiency;
- end-stage renal insufficiency (creatinine clearance (CC) < 15 ml/min);
- chronic heart failure of functional class IV (according to the NYHA classification.
How to use Rybelsus® Tablets?
- The drug Rybelsus® is a tablet for oral administration once a day.
- The drug should be taken on an empty stomach at any time of the day.
- The tablet must be swallowed whole, washed down with water (half a glass of water, which is equivalent to 120 ml). Tablets should not be divided, crushed or chewed, as it is not known whether this affects the absorption of semaglutide.
- Patients should wait at least 30 minutes before eating or drinking, or taking other oral medications. Failure to comply with this condition will lead to a decrease in the absorption of semaglutide.
How to choose a dosage for a Rybelsus® tablet?
- The initial dose of Rybelsus® is 3 mg once a day for one month. After 1 month of use, the dose should be increased to 7 mg once a day. To further improve glycemic control after at least 1 month of use at a dose of 7 mg once a day, the dose can be increased to a maintenance dose of 14 mg once a day.
- The maximum recommended dose of Rybelsus® for taking once a day is 14 mg. Taking two tablets of 7 mg to achieve the effect of a dosage of 14 mg has not been studied and is therefore not recommended.
- When using Rybelsus® in combination with metformin and / or an SGLT2 inhibitor or thiazolidinedione, therapy with metformin and / or an SGLT2 inhibitor or thiazolidinedione can be continued at the same doses.
- When using semaglutide in combination with a sulfonylurea derivative or insulin , a dose reduction of a sulfonylurea derivative or insulin should be provided in order to reduce the risk of hypoglycemia .
- To correct the dose of Rybelsus®, independent monitoring of blood glucose concentration is not required. Independent monitoring of glucose concentration in the blood is necessary to correct the dose of sulfonylurea and insulin, especially at the beginning of treatment with semaglutide and when reducing the dose of insulin. It is recommended to use a step-by-step approach to reducing the dose of insulin.
Overdose
During CI, overdose with Rybelsus® could be associated with gastrointestinal disorders. In case of overdose, appropriate symptomatic therapy is recommended, depending on the clinical manifestations and symptoms of the patient. Given the long half-life of semaglutide (approximately 1 week), an extended period of observation and treatment of overdose symptoms may be required. There is no specific antidote for overdose of the drug Rybelsus®.
Skipping a dose
If you miss a dose of the drug, you do not need to take the missed dose additionally, you should continue taking the drug as usual the next day.
What is the expiration date for Rybelsus® tablets?
30 months. Do not use after the expiration date.
How to store Rybelsus® tablets?
- Store at a temperature not exceeding 25°C in the original packaging (blister) to protect against moisture and light.
- Keep out of reach of children.
Contents
- 1 Buy Rybelsus® Semaglutide Tablets Online
- 1.1 Order Rybelsus® Semaglutide Tablets Tablets
- 1.2 Description of Rybelsus® Semaglutide Tablets
- 1.2.1 Pharmacodynamics
- 1.2.2 Fasting glycemia and postprandial glycemia
- 1.2.3 Glucagon secretion
- 1.2.4 Emptying the stomach
- 1.2.5 The concentration of lipids on an empty stomach and after meals
- 1.2.6 Clinical efficacy and safety
- 1.2.7 Monotherapy
- 1.2.7.1 Rybelsus® Semaglutide compared to empagliflozin, both in combination with metformin
- 1.2.7.2 Rybelsus® Semaglutide compared to sitagliptin, both in combination with metformin, or metformin together with sulfonylurea derivatives
- 1.2.7.3 Rybelsus® Semaglutide compared to liraglutide and placebo, all in combination with metformin or metformin with a type 2 sodium-dependent glucose transporter inhibitor (SGLT2)
- 1.2.7.4 Rybelsus® Semaglutide compared to sitagliptin, both in combination with metformin, SGLT2 inhibitors, sulfonylurea derivatives or thiazolidinediones.
- 1.2.7.5 Rybelsus® Semaglutide compared to placebo in combination with insulin
- 1.2.8 Assessment of the effect on the cardiovascular system
- 1.2.9 Body weight
- 1.2.10 Blood pressure
- 1.2.11 Children and teenagers
- 1.3 What are the Side effects of Rybelsus®?
- 1.4 What are the contraindications of Rybelsus?
- 1.5 How to use Rybelsus® Tablets?
- 1.6 How to choose a dosage for a Rybelsus® tablet?
- 1.7 What is the expiration date for Rybelsus® tablets?
- 1.8 How to store Rybelsus® tablets?