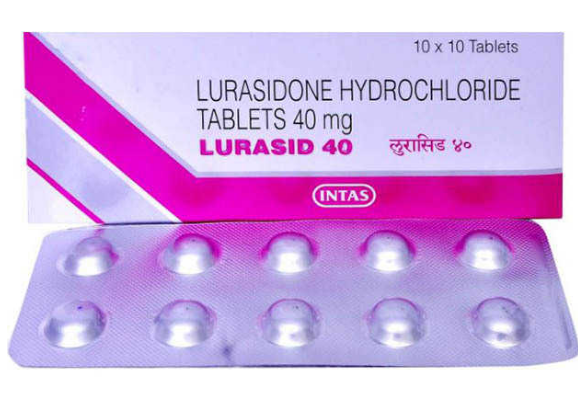
Buy Lurasid® 40 / 80 mg Online
- Brand: Latuda
- Generic name: Lurasid®
- Composition: Lurasidone Hydrochloride
- Power: 40mg, 80mg
- Drug form: Tablets
- Disease/Treatment: Schizophrenia, Bipolar disorder
- Manufacturer: Intas
- Country of Origin: India
Order Lurasid® Tablets

Lurasid® 40 / 80 mg is an antipsychotic drug. It works by altering the effects of chemicals in the brain.
Lurasid® is used to treat schizophrenia in adults and adolescents who are at least 13 years old.
Lurasid® is also used to treat episodes of depression in adults with bipolar disorder (manic depression).
- Packing: 10 tablets in 1 strip
- Minimum order quantity: 30 tablets
- Minimum price: $ 1.30 per tablet
- : 14-21 days
- Trackable service: 5-9 days
- Order from $150.00: Free AirMail shipping
How does Lurasid® Lurasidone work?
Lurasid® Lurasidone is a selective antagonist of dopamine and monoamine receptors, having a high affinity for D2-dopamine and 5HT2A- and 5HT7 serotonin receptors (Ki = 0.994, 0.47 and 0.495 nM, respectively). Lurazidone also blocks a2c- and a2a-adrenergic receptors (affinity 10.8 and 40.7 nM, respectively), has partial agonism to 5H1A-serotonin receptors with affinity 6.38 nM. Lurasid® Lurazidone does not bind to histamine and muscarinic receptors. The mechanism of action of the secondary active metabolite of lurazidone, ID-14283, is the same as that of lurazidone.
According to positron emission tomography, the use of lurazidone in the dose range from 9 to 74 mg (from 10 to 80 mg of lurazidone hydrochloride) in healthy volunteers led to a dose-dependent decrease in the binding of 11C-racloprid, a ligand of D2/D3 receptors, in the caudate nucleus, shell and ventral striatum.
What do I need to know before buying Lurasid® Lurasidone?
Lurasid® is not approved for use in psychotic conditions associated with dementia. Lurasidone may increase the risk of death in elderly people with dementia-related diseases.
Some young people have suicidal thoughts when they first take medications to treat depression. Watch for changes in mood or symptoms. Report any new or worsening symptoms to your doctor.
Before taking Lurasid®, tell your doctor if you have liver disease, kidney disease, heart disease, high blood pressure, heart rhythm problems, history of heart attack or stroke, high cholesterol or triglycerides, white blood cell count (WBC) Seizures, diabetes, Parkinson’s disease, swallowing problems or a history of breast cancer or suicidal thoughts.
Slide Show Bipolar Disorder Disorder – 12 Things You Need to Know
Some medications may interact with lurasidone and should not be used at the same time. Inform each of your healthcare providers about all medications you are currently using and any medication you are starting or stopping using.
While you are taking Lurasid®, you may be more sensitive to extreme temperatures, such as very hot or cold conditions. Avoid being too cold or overheated or dehydrated. Drink plenty of fluids, especially in hot weather and during exercise. It’s easier to become dangerously overheated and dehydrated while you’re taking this medicine.
Lurasid® can damage your thinking or reaction. Be careful if you drive or do anything that requires your attention. Avoid getting up too quickly from a sitting or lying position, or you may feel dizzy. Stand up slowly and steadily to prevent falling.
Alcohol consumption can cause certain side effects of lurasidone. Stop using this medicine and see your doctor right away if you have very stiff (stiff) muscles, high fever, sweating, confusion, rapid or pounding heartbeat, a feeling that you may disappear, trembling or twitching or uncontrolled movements of your eyes, lips, tongue, face, hands or feet.
Before taking Lurasid® 40 / 80 mg
You should not use Lurasid® if you are allergic to Lurasidone.
Some medications may interact with lurasidone and should not be used at the same time. Your doctor may change your treatment plan if you use some other medications, including:
- Antifungal medicine such as ketoconazole or voriconazole;
- An antibiotic such as clarithromycin or rifampin;
- Antiviral agent such as ritonavir;
- St. John’s Wort; or
- Withdrawal of medications such as carbamazepine or phenytoin.
Lurasid® is not approved for use in psychotic conditions associated with dementia. Lurasid® may increase the risk of death in elderly people with dementia-related diseases.
To make sure this medicine is safe for you, tell your doctor if you have ever had:
- Heart disease, high blood pressure;
- High cholesterol or triglycerides (a type of fat in the blood);
- Seizures or epilepsy;
- Liver or kidney diseases;
- White Blood cell Count (WBC);
- Diabetes or family history of diabetes;
- Abnormal hormone function tests (thyroid gland, pituitary gland);
- breast cancer;
- Depression or bipolar disease (unless you are taking Lurasid® to treat depressive episodes);
- Suicidal thoughts or actions; or hit.
Some young people have suicidal thoughts when they first take medications to treat depression. Your doctor should check your progress with regular visits. Your family or other caregivers should also be aware of changes in your mood or symptoms.
Taking antipsychotic medications during the last 3 months of pregnancy can cause problems in newborns, such as withdrawal symptoms, breathing problems, feeding problems, fussiness, tremors and limp or stiff muscles. However, you may have withdrawal symptoms or other problems if you stop taking the medication during pregnancy. If you become pregnant while taking Lurasid®, do not stop taking it without the advice of a doctor.
If you are pregnant, your name may be listed in the pregnancy registry. This should track the outcome of pregnancy and evaluate any effects of lurasidone on the baby.
It is not known whether lurasidone gets into breast milk or can harm a nursing baby. Tell your doctor if you are breastfeeding.
Lurasid® 40 / 80 mg is not approved for schizophrenia in someone under the age of 13. Lurasid® is not approved for depression in someone under the age of 18.
How to take Lurasid® 40 / 80 mg tablets?
Tablets Lurasid® 40 / 80 mg for oral administration.
The drug Lurasid® in film-coated tablets is taken orally once a day during meals. The expected exposure of lurazidone when taken on an empty stomach is significantly lower compared to the exposure when taken with a meal
Tablets of the drug Lurasid® are recommended to be swallowed whole to mask the bitter taste. Lurasid® 40 / 80 mg tablets should be taken every day at the same time to comply with the therapy regimen.
How to choose the dosage of Lurasid® 40 / 80 mg?
The recommended initial dose of Lurazidone Hydrochloride is 40 mg once a day. Titration of the dose is not required. The effect is achieved in the dose range from 40 to 160 mg per day. The dose of lurazidone can be increased by the doctor’s decision based on the clinical response. The maximum daily dose should not exceed 160 mg. Patients receiving a daily dose above 120 mg of lurazidone hydrochloride, who have interrupted treatment for more than 3 days, should resume taking, starting with 120 mg, followed by an increase in the dose of the drug to the optimal one. Patients receiving other doses can resume taking starting from the previous dose without increasing it.
Elderly patients (65 years and older)
Dose adjustment is not required in elderly patients with preserved renal function (creatinine clearance >80 ml/min). However, with a decrease in renal function in elderly patients, dose adjustment may be required depending on the degree of renal insufficiency.
There is limited data on the treatment of elderly patients with high doses of lurazidone. There is no information about the use of the drug Lurasid® at a dose of 160 mg for the treatment of elderly patients. Caution should be exercised when treating patients aged 65 years and older with high doses of the drug Lurasid®.
Patients with impaired renal function
Patients with mild renal insufficiency do not need to adjust the dose of lurazidone. In patients with moderate renal insufficiency (creatinine clearance > 30 and < 50 ml /min), severe (creatinine clearance > 15 and < 30 ml /min) and patients with terminal renal insufficiency (creatinine clearance < 15 ml/min), the recommended initial dose is 20 mg; the maximum dose it should not exceed 80 mg once a day. Lurasid® is used in patients with terminal renal insufficiency only if the potential benefit exceeds the potential risk of complications. Lurasid® should be used in patients with terminal renal insufficiency only with proper clinical control.
Patients with impaired liver function
Patients with mild hepatic insufficiency do not need to adjust the dose of Lurasid®.
Patients with moderate hepatic insufficiency (class B according to the Child-Pugh classification) and severe degrees of severity (class C according to the Child-Pugh classification) are recommended to adjust the dose. The recommended starting dose is 20 mg. The maximum daily dose in patients with moderate hepatic insufficiency should not exceed 80 mg, in patients with severe hepatic insufficiency should not exceed 40 mg once a day.
Children
The safety and efficacy of Lurasid® in children under the age of 18 have not been established.
Dose adjustment depending on the interaction
With the simultaneous use of Lurasid® with moderate inhibitors of the CYP3A4 iso-enzyme, the recommended initial dose is 20 mg, the maximum daily dose should not exceed 80 mg. Dose adjustment may be required when used in combination with mild and moderate inducers of the CYP3A4 isoenzyme. With regard to simultaneous use with strong inhibitors and inducers of the CYP3A4 isoenzyme.
Transfer of patients to treatment with other antipsychotic drugs
The pharmacodynamics and pharmacokinetics of various antipsychotic drugs are not the same, so doctors should carefully monitor the condition of patients when transferring them from one antipsychotic drug to another.
What should be avoided when taking Lurasid®?
- It’s easier to become dangerously overheated and dehydrated while you’re taking Lurasid®. Taking plenty of fluids, especially in hot weather and during exercise. You may also be more sensitive to extreme temperatures (hot or cold).
- Grapefruit and grapefruit juice can interact with lurasidone and lead to undesirable side effects. Avoid using grapefruit products while taking Lurasid®.
- Lurasid® can damage your thinking or reaction. Be careful if you drive or do anything that requires your attention. Avoid getting up too quickly from a sitting or lying position, or you may feel dizzy. Stand up slowly and steadily to prevent falling.
- Avoid drinking alcohol. Dangerous side effects may occur.
What are the Side effects of Lurasid®?
Get emergency medical attention if you have signs of an allergic reaction to Lurasid®:
- hives;
- difficulty breathing;
- Swelling of the face, lips, tongue or throat.
Report any new or worsening symptoms to your doctor, such as:
- mood or behavior changes,
- anxiety,
- panic attacks,
- sleep problems or if you feel impulsive,
- irritable,
- agitated,
- hostile,
- aggressive,
- anxious,
- hyperactive (mentally or physically),
- more depressed or thoughts of suicide or harm to myself.
High doses or prolonged use of Lurasid® can cause serious movement disorders that cannot be reversible. Symptoms of this disorder include uncontrolled movements of the muscles of your lips, tongue, eyes, face, arms or legs. The longer you take this medicine, the more likely you are to develop a serious movement disorder. The risk of this side effect is higher in women and the elderly.
Call your doctor immediately if you have:
- Irregular menstrual periods, breast or vaginal changes, nipple loss;
- Dizziness, fainting, fast or slow heartbeat;
- Problems with swallowing;
- Seizure (convulsions);
- Disorders of blood cells – sudden weakness or bad feeling, fever, chills, sore throat, mouth ulcers, swollen gums, pain when swallowing, skin ulcers, cold or flu symptoms, cough, difficulty breathing;
- High blood sugar – increased thirst, increased urination, hunger, dry mouth, the smell of fruit breath, drowsiness, dry skin, blurred vision, weight loss; or
- A strong reaction of the nervous system – very stiff (rigid) muscles, high fever, sweating, confusion, rapid or uneven heartbeat, tremor, feeling that you can disappear.
Common side effects of Lurasid® may include:
- weight gain;
- drowsiness;
- Nausea, vomiting;
- Feeling anxious or unable to sit still; or
- Tremor, muscle stiffness, problems with muscle movement.
What are the instructions when taking Lurasid® 40 / 80 mg tablets?
When treated with antipsychotic drugs, improvement in the clinical condition of the patient should be expected within a few days to several weeks. Proper monitoring of the patient’s condition during this period is necessary.
Suicidal intentions
The tendency to suicidal thoughts and attempts is characteristic of patients with psychosis. There are reports of similar cases at the beginning of therapy or when replacing an antipsychotic drug. Therefore, drug antipsychotic therapy should be carried out under close medical supervision.
Parkinson’s disease
Caution should be exercised when using antipsychotic drugs in patients with Parkinson’s disease, because this group of patients has increased sensitivity to antipsychotic drugs, and the risk of exacerbation of parkinsonism symptoms is increased. The drug Lurasid® can be used in patients with Parkinson’s disease only in cases where the potential benefit exceeds the possible risk to the patient.
Extrapyramidal symptoms (EPS)
Drugs that have the properties of dopamine receptor antagonists can cause undesirable extrapyramidal disorders, including rigidity, tremor, mask-like face, dystonia, salivation, impaired posture and gait. According to placebo-controlled CI in adult patients with schizophrenia, the use of lurazidone was accompanied by an increase in the incidence of extrapyramidal symptoms compared with placebo.
Late dyskinesia
Drugs with dopamine receptor antagonist properties can cause tardive dyskinesia, which is characterized by rhythmic involuntary movements, especially of the tongue and/or face. If symptoms of tardive dyskinesia appear, a decision should be made on the expediency of canceling all antipsychotic drugs, including lurazidone.
Cardiovascular diseases/prolongation of the QT interval
Caution should be exercised when prescribing lurazidone to patients with diagnosed cardiovascular diseases or an extended QT interval in close relatives, hypokalemia, as well as when used simultaneously with other medications that lengthen the QT interval.
Convulsions
Caution should be exercised when prescribing lurazidone to patients with a history of seizures or other conditions that potentially lower the threshold of convulsive activity.
Malignant neuroleptic syndrome
Cases of the development of malignant neuroleptic syndrome characterized by hyperthermia, muscle rigidity, instability of autonomous functions, impaired consciousness and increased activity of creatyphosphokinase in the blood when using antipsychotic drugs, including lurazidone, have been reported. In addition, the development of myoglobinuria (rhabdomyolysis) and acute renal failure is possible. In such cases, it is necessary to cancel all antipsychotic drugs, including lurazidone.
Elderly patients with dementia
In elderly patients with dementia, the use of lurazidone has not been studied.
Total mortality rate
According to a meta-analysis of 17 controlled CI in elderly patients with dementia treated with other atypical antipsychotic drugs, including risperidone, aripiprazole, olanzapine and queatipine, there was an increase in the overall mortality rate compared with placebo.
Cerebrovascular disorders
According to the data of randomized placebo-controlled CI in patients with dementia treated with atypical antipsychotic drugs, including risperidone, aripiprazole and olanzapine, there was an approximately 3-fold increase in the risk of cerebrovascular adverse reactions, the mechanism of which is unknown. It is impossible to exclude an increased risk of cerebrovascular disorders for other antipsychotic drugs or other groups of patients. Lurazidone should be used with caution in elderly patients with dementia who have risk factors for stroke.
Venous thromboembolism
When using antipsychotic drugs, cases of venous thromboembolism have been noted. Since patients taking antipsychotic drugs often have a risk of developing venous thromboembolism, all possible risk factors for venous thromboembolic complications should be identified before and during treatment with lurazidone, and preventive measures should be taken/
Hyperprolactinemia
Lurazidone increases the concentration of prolactin, as it is an antagonist of D2-dopamine receptors.
Weight gain
When taking atypical antipsychotic drugs, an increase in body weight was observed. It is recommended to control body weight.
Hyperglycemia
According to CI, the use of lurazidone in rare cases was accompanied by the development of adverse reactions associated with changes in glucose concentration, for example, hyperglycemia. Proper clinical monitoring is recommended in the treatment of patients with diabetes mellitus and risk factors for the development of diabetes mellitus.
Orthostatic hypotension/fainting
Orthostatic hypotension may develop due to the presence of the properties of an α1-adrenergic receptor antagonist in lurazidone. Proper monitoring of the symptoms of orthostatic hypotension in patients at risk of lowering blood pressure is recommended.
Kidney failure
It is recommended to adjust the dose of lurazidone in patients with moderate, severe and terminal renal insufficiency. There is no data on the use of lurazidone in patients with terminal renal insufficiency, therefore lurazidone can be used only in cases where the potential benefit exceeds the possible risk. Treatment of patients with end-stage renal insufficiency with lurazido should be carried out under proper control of the patient’s condition.
Liver failure
It is recommended to adjust the dose of lurazidone in patients with moderate and severe hepatic insufficiency (class B and C according to the Child-Pugh classification). Proper monitoring of the patient’s condition is required when using lurazidone in patients with severe hepatic insufficiency.
Interaction with grapefruit juice
Grapefruit juice should be avoided when treating with lurazidone .
The remaining unused drug must be disposed of in accordance with local requirements.
Contents
- 1 Buy Lurasid® 40 / 80 mg Online
- 1.1 Order Lurasid® Tablets
- 1.2 How does Lurasid® Lurasidone work?
- 1.3 What do I need to know before buying Lurasid® Lurasidone?
- 1.4 Before taking Lurasid® 40 / 80 mg
- 1.5 How to take Lurasid® 40 / 80 mg tablets?
- 1.6 How to choose the dosage of Lurasid® 40 / 80 mg?
- 1.7 What should be avoided when taking Lurasid®?
- 1.8 What are the Side effects of Lurasid®?
- 1.9 What are the instructions when taking Lurasid® 40 / 80 mg tablets?
- 1.9.1 Suicidal intentions
- 1.9.2 Parkinson’s disease
- 1.9.3 Extrapyramidal symptoms (EPS)
- 1.9.4 Late dyskinesia
- 1.9.5 Cardiovascular diseases/prolongation of the QT interval
- 1.9.6 Convulsions
- 1.9.7 Malignant neuroleptic syndrome
- 1.9.8 Elderly patients with dementia
- 1.9.9 Total mortality rate
- 1.9.10 Cerebrovascular disorders
- 1.9.11 Venous thromboembolism
- 1.9.12 Hyperprolactinemia
- 1.9.13 Weight gain
- 1.9.14 Hyperglycemia
- 1.9.15 Orthostatic hypotension/fainting
- 1.9.16 Kidney failure
- 1.9.17 Liver failure
- 1.9.18 Interaction with grapefruit juice