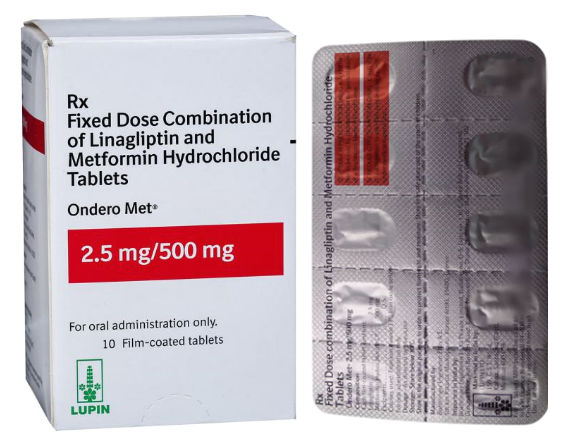
Buy Ondero Met® 2.5 mg / 500 mg Online
- Brand: Jentadueto
- Generic name: Ondero Met® 2.5 mg / 500 mg
- Composition: Metformin hydrochloride (500 mg) & Linagliptin (2.5 mg)
- Drug form: Tablets
- Disease/Treatment: used in adults with type 2 diabetes to improve blood glucose control
- Manufacturer: LUPIN LTD
- Country of Origin: India
Order Ondero Met® 2.5 mg / 500 mg Tablets
- Packing: 10 tablets in 1 strip
- Minimum order quantity: 30 tablets
- Minimum price: $ 3.00 per tablet
- : 14-21 days
- Trackable service: 5-9 days
- Order from $150.00: Free AirMail shipping
Ondero Met® 2.5 mg / 500 mg Tablets are used in type 2 diabetes mellitus:
- in order to improve glycemic control (in combination with diet and exercise) in cases where simultaneous use of linagliptin and metformin is advisable: in patients whose treatment with metformin alone is not effective enough, or in patients who are already receiving a combination of linagliptin and metformin in the form of separate drugs with a good effect;
- in combination with sulfonylurea derivatives (triple combination therapy) in addition to diet and exercise for patients whose treatment with metformin and sulfonylurea derivatives in maximum tolerated doses is not effective enough.
Before using the drug Ondero Met® 2.5 mg / 500 mg, a doctor’s consultation is necessary.
What is Ondero Met® 2.5 mg / 500 mg?
Ondero Met® 2.5 mg / 500 mg contains a combination of linagliptin and metformin. Linagliptin and metformin are oral diabetes medications that help control blood sugar levels. Metformin works by reducing the production of glucose (sugar) in the liver and reducing the absorption of glucose in the intestine. Linagliptin works by regulating the levels of insulin that your body produces after eating.
Ondero Met® 2.5 mg / 500 mg is used together with diet and exercise to improve blood sugar control in adults with type 2 diabetes.
Ondero Met® 2.5 mg / 500 mg is not intended for the treatment of type 1 diabetes.
How to take Ondero Met® 2.5 mg / 500 mg tablets?
- Ondero Met® 2.5 mg / 500 mg for oral administration.
- The recommended dose is 1 tablet of Ondero Met® 2.5 mg / 500 mg 2 times / day.
- Ondero Met® 2.5 mg / 500 mg should be taken with meals in order to reduce gastrointestinal adverse reactions caused by metformin.
Dosage of Ondero Met® 2.5 mg / 500 mg
The dosage should be selected individually based on the patient’s current treatment regimen, its effectiveness and tolerability. The maximum recommended daily dose of Ondero Met® 2.5 mg / 500 mg is 5 mg of linagliptin and 2000 mg of metformin.
When transferring from the combined use of linagliptin and metformin to the same fixed combination, the Ondero Met® 2.5 mg / 500 mg is recommended to be used in such a way that the doses of linagliptin and metformin remain unchanged.
In patients with inadequate control of diabetes mellitus on the background of dual combination therapy with the use of maximum tolerated doses of metformin and a sulfonylurea derivative, the Ondero Met® 2.5 mg / 500 mg Pills is usually recommended to be used in such a way that the dose of linagliptin is 2.5 mg twice a day (daily dose of 5 mg), and the dose of metformin remains unchanged. When using the drug Ondero Met® 2.5 mg / 500 mg in combination with a sulfonylurea derivative, a lower dose of a sulfonylurea derivative may be required to reduce the risk of hypoglycemia.
In patients with inadequate control of diabetes mellitus on the background of double combination therapy with the use of insulin and the maximum tolerated dose of metformin, the drug Ondero Met® 2.5 mg / 500 mg is usually recommended to be used in such a way that the dose of linagliptin is 2.5 mg twice a day (daily dose of 5 mg), and the dose of metformin remains unchanged. When using the Ondero Met® 2.5 mg / 500 mg in combination with insulin, a lower dose of insulin may be required to reduce the risk of hypoglycemia.
Overdose
Symptoms: During controlled clinical trials conducted in healthy volunteers, single doses of linagliptin reaching 600 mg (equivalent to 120 times the recommended dose) were well tolerated. A person has no experience of using the drug in doses exceeding 600 mg.
When metformin was used in doses reaching 85 g, hypoglycemia was not observed, there were cases of lactic acidosis. A significant overdose of metformin can lead to lactic acidosis.
Therapy: In case of overdose, the usual supportive measures are used, for example, removal of the unabsorbed drug from the gastrointestinal tract, symptomatic therapy. Lactic acidosis belongs to the category of urgent medical conditions, treatment in such cases should be carried out in a hospital.
The most effective method of removing lactate and metformin is hemodialysis.
What happens if I miss a dose?
Take the missed dose as soon as you remember (be sure to take the medicine with food). Skip the missed dose if it’s almost time for your next scheduled dose. Do not take additional medication to make up for the missed dose.
Before buying Ondero Met® 2.5 mg / 500 mg medicine
You should not use Onder Met® 2.5 mg / 500 mg if you are allergic to metformin (Actoplus Met, Avandamet, Fortamet, Glucophage, Riomet) or linagliptin, or:
- If you have ever had a severe allergic reaction (breathing problems, swelling, severe skin rash) to linagliptin (Tradjenta);
- If you have severe kidney disease; or
- If you have diabetic ketoacidosis (consult a doctor for treatment).
Some people taking metformin develop a severe condition called lactic acidosis. This may be more likely if you have liver or kidney disease, heart failure, heart attack or stroke, severe infection, if you are over 65, if you are dehydrated or if you drink a lot of alcohol. Talk to your doctor about your risk.
What are the indications for the use of Ondero Met® 2.5 mg / 500 mg?
The drug Ondero Met® 2.5 mg / 500 mg is indicated in patients with type 2 diabetes mellitus as a supplement to diet and exercise in order to improve glycemic control in cases where simultaneous use of linagliptin and metformin is advisable: in patients whose treatment with metformin alone is not effective enough, or in patients who already receive a combination of linagliptin and metformin in the form of separate drugs with good effect.
The drug Ondero Met® 2.5 mg / 500 mg can be prescribed in combination with sulfonylureas (triple combination therapy) in addition to diet and exercise to patients in whom the use of metformin and sulfonylureas derivatives in maximum tolerated doses is not effective enough.
The drug Ondero Met® 2.5 mg / 500 mg can be prescribed in combination with insulin (triple combination therapy) in addition to diet and exercise to patients in whom the use of metformin and insulin does not provide adequate glycemic control.
How does Ondero Met® 2.5 mg / 500 mg work?
Ondero Met® 2.5 mg / 500 mg is a combination of two hypoglycemic drugs with complementary mechanisms of action to improve glycemic control in patients with type 2 diabetes mellitus: linagliptin, a dipeptidyl peptidase–4 (DPP-4) inhibitor, and metformin hydrochloride, a representative of the biguanide class.
Linagliptin
Linagliptin is an inhibitor of the enzyme dipeptidyl peptidase-4 (DPP-4), which is involved in the inactivation of hormones incretins – glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). These hormones are rapidly destroyed by the enzyme DPP-4. Both of these hormones are involved in the physiological regulation of glucose homeostasis. The basal level of incretin secretion during the day is low, it rises rapidly after eating. GLP-1 and GIP enhance insulin biosynthesis and its secretion by pancreatic beta cells at normal and elevated blood glucose levels. In addition, GLP-1 reduces the secretion of glucagon by pancreatic alpha cells, which leads to a decrease in glucose production in the liver. Linagliptin binds actively and reversibly to DPP-4, which causes a steady increase in incretin levels and a long-term preservation of their activity. Linagliptin increases glucose-dependent insulin secretion and reduces glucagon secretion, resulting in improved glucose homeostasis. Linagliptin binds to DPP-4 selectively, in vitro its selectivity for DPP-4 is higher than the selectivity for DPP-8 or DPP-9 by more than 10,000 times.
Metformin
Metformin hydrochloride is a hypoglycemic biguanide that reduces both basal and postprandial glucose concentrations in blood plasma. It does not stimulate insulin secretion and therefore does not cause hypoglycemia. Metformin hydrochloride has 3 mechanisms of action:
- reduces the production of glucose by the liver by inhibiting gluconeogenesis and glycogenolysis;
- in muscles increases the sensitivity of peripheral receptors to insulin and glucose utilization by cells;
- slows down the absorption of glucose in the intestine.
Metformin hydrochloride stimulates the synthesis of intracellular glycogen by acting on glycogen synthase. Increases the transport capacity of all types of currently known membrane glucose transporters. In humans, regardless of the effect on the level of glycemia, metformin hydrochloride had a beneficial effect on lipid metabolism. This effect included a decrease in the level of total cholesterol, LDL cholesterol and triglycerides and was demonstrated in controlled medium- and long-term clinical studies of the use of therapeutic doses of metformin hydrochloride.
What are the contraindications for Ondero Met® 2.5 mg / 500 mg tablets?
- Hypersensitivity to any component of the drug;
- Type 1 diabetes mellitus;
- Diabetic ketoacidosis;
- Diabetic precoma;
- Renal insufficiency or impaired renal function (creatinine clearance less than 60 ml/min);
- Acute conditions occurring with the risk of developing renal dysfunction: dehydration (with diarrhea, vomiting), severe infectious diseases, shock;
- Clinically pronounced manifestations of acute or chronic diseases that can lead to the development of tissue hypoxia (including cardiac or respiratory failure, acute myocardial infarction);
- Liver failure, impaired liver function;
- Acute alcohol intoxication;
- Chronic alcoholism;
- Children under 18 years of age (due to insufficient data on efficacy and safety);
- Pregnancy and breastfeeding (due to insufficient data on efficacy and safety);
- Extensive surgical operations and injuries when insulin therapy is indicated;
- Use for less than 48 hours before and for 48 hours after conducting radioisotope or X-ray examinations with the introduction of an iodine-containing contrast agent (see the section “Interaction with other drugs”);
- Lactic acidosis (including a history);
- Compliance with a hypocaloric diet (less than 1000 kcal / day).
What are the side effects of Ondero Met® 2.5 mg / 500 mg?
The safety of linagliptin, administered at a dose of 2.5 mg twice a day (or at a bioequivalent dose of 5 mg once a day) in combination with metformin, has been studied in more than 6800 patients with type 2 diabetes mellitus. In placebo-controlled trials, more than 1,800 patients received a therapeutic dose of linagliptin 2.5 mg twice daily (or a bioequivalent dose of 5 mg once daily) in combination with metformin for ≥12/24 weeks.
According to the results of a combined analysis of seven placebo-controlled trials, the number of cases of side effects in patients receiving placebo and metformin was comparable to the number of cases in patients taking linagliptin 2.5 mg and metformin (54.3% and 49.0%). The number of cases of discontinuation of therapy due to side effects was comparable in patients receiving placebo and metformin (3.8%) and in patients taking a combination of linagliptin and metformin (2.9%).
The most frequently reported side effect associated with the use of a combination of linagliptin and metformin was diarrhea (1.6%), observed less frequently than when taking a combination of metformin and placebo (2.4%).
When using Ondero Met® 2.5 mg / 500 mg in combination with a sulfonylurea derivative, hypoglycemia may develop (≥ 1 case per 10 patients).
Precautions
Ondero Met® 2.5 mg / 500 mg should not be prescribed to patients with type 1 diabetes mellitus.
Hypoglycemia
The number of cases of hypoglycemia with the addition of sulfonylurea derivatives to linagliptin and metformin therapy increased compared to placebo.
Sulfonylurea derivatives and insulin can cause hypoglycemia. Therefore, caution should be exercised when using Ondero Met® 2.5 mg / 500 mg in combination with a sulfonylurea derivative and/ or insulin. It may be necessary to reduce the dose of a sulfonylurea derivative or insulin. Hypoglycemia is not a side effect of the use of linagliptin, metformin or their combination. According to clinical trials, the number of cases of hypoglycemia in patients taking linagliptin in combination with metformin or metformin alone was low.
Lactic acidosis
Lactic acidosis is a very rare but serious metabolic complication, most often occurring with acute deterioration of kidney function or cardiorespiratory diseases or sepsis. Metformin accumulation occurs with acute deterioration of kidney function and increases the risk of lactic acidosis.
In case of dehydration (severe diarrhea or vomiting, fever or decreased fluid intake), metformin should be temporarily discontinued and it is recommended to consult your doctor.
Patients receiving metformin should be cautiously prescribed medications that can dramatically worsen kidney function (for example, antihypertensive agents, diuretics and NSAIDs). Other risk factors for lactic acidosis are excessive alcohol consumption, impaired liver function, inadequately controlled diabetes, ketosis, prolonged hunger, any conditions associated with hypoxia, as well as concomitant use of medications that can cause lactic acidosis.
Patients and/or caregivers should be informed about the risk of lactic acidosis. Lactic acidosis is characterized by acidotic dyspnea, abdominal pain, muscle cramps, asthenia and hypothermia, accompanied by coma. In case of suspicious symptoms, the patient should stop taking metformin and immediately consult a doctor. Diagnostic laboratory indicators: a decrease in blood pH (<7.35), an increase in plasma lactate levels (>5 mmol/ L) and an increase in the ratio of the anion interval and lactate /pyruvate.
The use of iodized contrast agents
Intravascular administration of iodized contrast agents in radiological studies can lead to renal failure, which can cause the accumulation of metformin and increase the risk of lactic acidosis. In this regard, the use of the drug should be stopped in advance or during such studies and resumed 48 hours after the end of the studies and only after receiving the results of a re-evaluation of kidney function, indicating that there are no deviations from the norm.
Impaired renal function
GFR should be evaluated before the start of treatment and regularly during treatment. Metformin is contraindicated in patients with GFR <30 ml/min. and should be temporarily discontinued in the presence of conditions that alter kidney function.
Heart function
Patients with heart failure have an increased risk of hypoxia and renal failure. Ondero Met® 2.5 mg / 500 mg can be taken with stable chronic heart failure, provided that the function of the heart and kidneys is regularly monitored.
In patients with acute and decompensated heart failure, the use of Ondero Met® 2.5 mg / 500 mg is contraindicated.
Surgical intervention
Since Ondero Met® 2.5 mg / 500 mg contains metformin hydrochloride, the drug should be discontinued 48 hours before the planned surgical intervention using general, spinal or epidural anesthesia. Its use can be continued no earlier than 48 hours after surgery and only if the results of a repeated assessment of kidney function are obtained, indicating that there are no deviations from the norm.
Elderly patients
When treating patients over 80 years of age, caution should be exercised.
Changes in the clinical status of patients with previously diagnosed controlled type 2 diabetes mellitus.
Since Ondero Met® 2.5 mg / 500 mg contains metformin, if laboratory deviations from the norm or a clinical disease are detected in patients with previously adequately controlled type 2 diabetes mellitus (especially with an uncertain diagnosis and an unclear clinical picture), it is necessary to immediately conduct an examination for the presence of ketoacidosis or lactic acidosis. The examination should include the determination of electrolytes and ketones in the blood serum, blood glucose and, if necessary, blood pH, lactate, pyruvate in the blood and the concentration of metformin. In case of acidosis in any form, taking Ondero Met® 2.5 mg / 500 mg should be stopped immediately and appropriate treatment should be prescribed.
Acute pancreatitis
The use of DPP-4 inhibitors was associated with an increased risk of acute pancreatitis. During the post-registration use of linagliptin, spontaneous reports of the development of acute pancreatitis were received. Patients should be informed about the characteristic symptoms of acute pancreatitis. If pancreatitis is suspected, the use of Ondero Met® 2.5 mg / 500 mg should be discontinued. If the diagnosis of acute pancreatitis is confirmed, the repeated appointment of Ondero Met® 2.5 mg / 500 mg is excluded. Caution should be exercised when prescribing the drug to patients with a history of pancreatitis.
Bullous pemphigoid
Postmarketing reports of bullous pemphigoid have been received in patients taking linagliptin. If bullous pemphigoid is suspected, the use of Ondero Met® 2.5 mg / 500 mg should be discontinued.
Contents
- 1 Buy Ondero Met® 2.5 mg / 500 mg Online
- 1.1 Order Ondero Met® 2.5 mg / 500 mg Tablets
- 1.2 What is Ondero Met® 2.5 mg / 500 mg?
- 1.3 How to take Ondero Met® 2.5 mg / 500 mg tablets?
- 1.4 Dosage of Ondero Met® 2.5 mg / 500 mg
- 1.5 Before buying Ondero Met® 2.5 mg / 500 mg medicine
- 1.6 What are the indications for the use of Ondero Met® 2.5 mg / 500 mg?
- 1.7 How does Ondero Met® 2.5 mg / 500 mg work?
- 1.8 What are the contraindications for Ondero Met® 2.5 mg / 500 mg tablets?
- 1.9 What are the side effects of Ondero Met® 2.5 mg / 500 mg?
- 1.10 Precautions